Background: Pyruvate Kinase (PK) deficiency is a rare congenital hemolytic anemia with an estimated prevalence of 3.2 to 8.5 cases per million in Western populations. Although research is limited, PK deficiency has a wide-ranging impact on quality of life (QoL) physically and psychosocially. Patients often feel that information in these domains in not adequately captured nor the interconnections between them understood during different life phases.
To address this gap, the PK Deficiency Advocacy Advisory Council (AAC; formed and funded by Agios Pharmaceuticals), a multi-disciplinary group of patients, carers, advocates, and health care providers (HCPs), developed the PK Deficiency Life Phase Model that summarizes the physical and psychosocial symptoms individuals and their carers may face at specific life phases.
Objective: Provide HCPs, patients, and carers a guiding source of information to further understand the potential physical and psychosocial impacts of PK deficiency through a life phase model based on the lived experience of patients and carers.
Methods: Life phases were based on published literatureand AAC input (N=2 HCPs, N=4 patients, N=1 advocate and N=1 carer). Lived experiences were collected in AAC workshops and sorted into either physical or psychosocial domains/ themes and assigned to specific life phase(s). The impact of each experience on patients in each life phase was scored on a high to low frequency scale based on AAC experiences. This content formed the Life Phase Model which was verified through several subsequent patient advocacy research steps.
A targeted literature review of physical and psychosocial topics was also conducted to refine the model, which was further supplemented with data from Agios' Peak Registry. The Model was pressure tested in three focus groups representing young adult patients aged 18 to 25 (N=9), mature patients aged over 25 (N=14), or carers (N=7) from nine countries. Additional input was collected in in-depth interviews (N=4). Research was conducted in accordance with British Healthcare Business Intelligence Association Legal and Ethical Guidelines, as well as guidelines established by UK Market Research Society. These steps were taken to ensure diversity, accuracy, and inclusivity of experiences from the whole community.
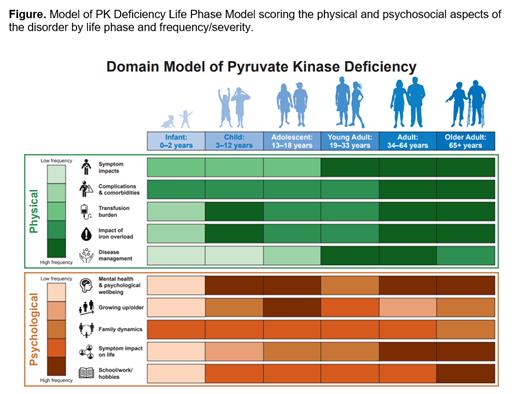
Results: Physical themes associated with each life phase included symptoms, complications/comorbidities, impacts of transfusions, iron overload and disease management options. Psychological themes included disease impact on mental health and psychological wellbeing, family dynamics, aging, school and work, symptom impact on daily life, and the future. Most supportive literature was relevant to physical domains (N=18); few reports were available to support the psychological themes (N=9).
These themes were used to develop a PK Deficiency Life Phase Model (Figure). Information from the literature, AAC patient and carers members, and focus group participants were used to determine the degree of impact or burden of each physical and psychological theme at each life stage. Higher impact was depicted with a darker shade in the model. The resulting model provides a well-grounded snapshot of the implications of living with PK deficiency and patient/carer perspective of how each category affects the individual patient population.
For most physical and psychological themes, frequency tends to increase with age. For example, the impact of disease symptoms was most severe for young adults, adults, and older adults. Complications and comorbidities associated with PK deficiency appear to affect individuals of all ages similarly. The impact of the disease on mental health increases with age. Adolescents struggled with challenges associated with growing up with a chronic condition. All age groups noticed the impact of PK deficiency on school, work, and/or hobbies.
Conclusions: The PK Deficiency Life Phase Model captures the psychological and physical burden of disease from the literature and directly from patients and carers. Hematologists can use this model to better understand how the spectrum of disease burden impacts patients and carers over time, further serving as a source of information for patients, carers, and healthcare professionals that support this rare disease community. Future research efforts can build on this qualitative work to further understand PK Deficiency patient experience over time.
Disclosures
Grace:Sanofi: Consultancy; Agios: Consultancy, Research Funding; Novartis: Research Funding; Sobi: Research Funding. Cannon:Agios Pharmaceuticals: Consultancy, Honoraria. Eijgenraam:Agios: Consultancy, Honoraria. Lander:Agios Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees. Miller D'Angelo:Agios: Consultancy, Honoraria. Morris:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Davis:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Patel:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Schryver:Agios: Consultancy, Honoraria. Watson:Agios Pharmaceuticals: Consultancy, Honoraria. Barcellini:Novartis: Consultancy, Honoraria, Speakers Bureau; Alexion, AstraZeneca Rare Disease: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.